Glaucoma Research
Dr. Adriana Di Polo’s laboratory employs pre-clinical models of acute and chronic optic nerve damage to explore molecular signals governing the survival of retinal ganglion cells following injury. Additionally, we examine interactions between neurons and glia, as well as vascular abnormalities, contributing to neurodegeneration in glaucoma.
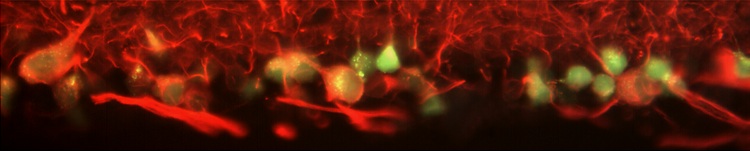
NEURONAL SURVIVAL AND REGENERATION
Dr. Adriana Di Polo’s laboratory is dedicated to uncovering molecular signals that govern the survival and regeneration of retinal ganglion cells in injured eyes. Utilizing pre-clinical models of glaucoma, we are presently investigating the signaling mechanisms involved in retinal ganglion cell death, dendrite retraction and regrowth, axon degeneration, synaptic loss, and dysfunction. Our laboratory actively contributes to the pre-clinical development of compounds currently undergoing clinical trials for ophthalmological purposes. Dr. Di Polo’s laboratory aims to leverage this knowledge to devise clinically viable strategies aimed at enhancing the protection and regeneration of retinal ganglion cells in individuals with glaucoma.
NEURON-GLIA INTERACTIONS
Glial cells, including microglia, play critical roles in maintaining retinal function. Recent data demonstrate an important neuroinflammatory component in glaucoma characterized by reactive gliosis and upregulation of proinflammatory cytokines. We are actively investigating how reactive glia, including microglia, contribute to retinal ganglion cell damage in glaucoma.
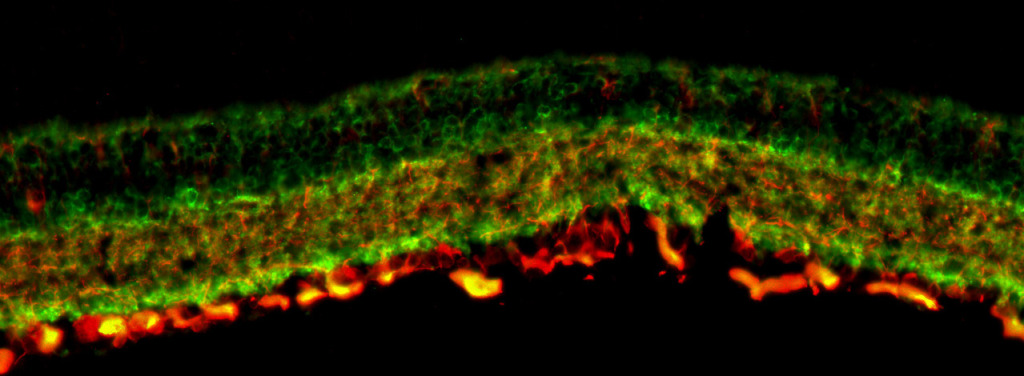
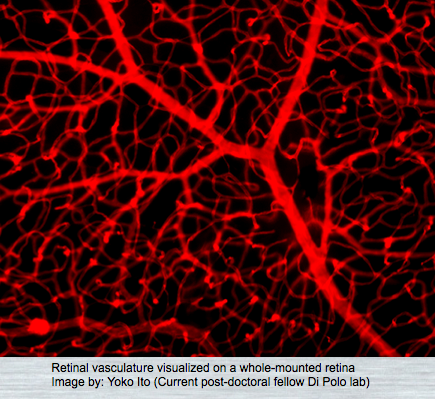
VASCULAR DYSFUNCTION
The retina, being one of the most metabolically active tissues in the body, necessitates precise control over its blood supply to fulfill its high oxygen demand. Vascular deficits are hypothesized to play a role in the death of retinal ganglion cells and the progression of glaucoma. Our research aims to elucidate the mechanisms underlying neurovascular dysfunction in glaucoma, involving not only retinal ganglion cells but also pericytes and endothelial cells.
